SEMINAR ON "QUALITY ASSURANCE AND QUALITY CONTROL IN THE LABORATORY SERVICES"
he Association of Biomedical Analysts (ABA) organised a seminar on the above theme at the Gold Crest Hotel on Sunday 7th May 2000. Honourable K. Deerpalsingh, Minister of Health & Quality of Life, as well as the top officials of his ministry namely Dr Sungkur, Chief Medical Officer, Mr Hosseny , permanent assistant secretary, Dr Mohit, Director of the Mauritius Institue of Health (MIH) and Dr Gopee, Representative of World Health Organisation (WHO) in Mauritius were present on this occasion.
In his speech, Mr K.T Ramessur, President of ABA, presented the association and announced that it was celebrating its 5th Anniversary this year. He said that there is a real need for the upgrading as well as for further education for the medical laboratory technicians. He added that in spite of reports which date for more than 20 years, no institute comparable to the Institute of Biomedical Science (IBMS) has been set up. Hence, at the minister's request, ABA prepared a report for the upgrading of our Diploma into a Bsc Biomedical Sciences course. The President of ABA handed over a copy of the report to the minister during the opening ceremony.
Dr (Mrs) Jeebun, consultant pathologist, in her speech said that the chosen topic for this seminar should be of interest to those working in a diagnostic laboratory. She briefly described the Central Health Laboratory as the main diagnostic laboratory which offers tests both in the routine and emergency services to the public at large on a 24-hour basis. She pointed out that the objective of the seminar was to make the laboratory staff aware of good laboratory practice and that quality assurance in the laboratory services will enhance the quality of health care. She also talked about a workshop that she attended in October 1998 in Addis-Abeba, there she realised that the laboratory services in Mauritius were ahead in most aspects concerning quality control and quality assurance than these 16 english-speaking African countries. In the future, she said that the Central Health Laboratory must become a reference laboratory for the region and added that the virology section is already a reference laboratory for the sexually transmitted diseases. Another target that must be achieved is the accreditation of the laboratory services from MAURITAS, an accreditating body in Mauritius. Finally she stressed on the laboratory reorganisation where the actual diplomates must be converted into Bsc holders such that a better trained staff is available to the laboratory services.
The Minister of Health, Honourable Kishore Deerpalsingh, in his speech emphasised on the fact that quality should be of utmost importance to all of the health sector personnel because patient satisfaction is fundamental. He said that the negotiations with Curtin University (Australia) have reached an advanced stage and that personally he was in favour of a Bsc Biomedical Sciences. In this context, he assured the laboratory personnel of his full support and added that this course will be subsidised by the ministry.
Coming to the seminar itself, it can be said that the objectives of such an event have been reached. At least 40 professionals from both the public and private sectors had participated in this event on that day. It should be pointed out that it is the first time
that such an event was organised in Mauritius. Among the participants, we noted the presence of Dr Usha Mehta who is a microbiologist from All India Institute of Medical Sciences.
It is a fact that quality should be present in the everyday life of the laboratory personnnel because the consequences of poor quality can be disastreous as shown below:
|
EVENT |
CONSEQUENCES |
|
Inappropriate action |
Overinvestigation Treatment/ Mistreatment |
|
Inappropriate Action |
Lack of investigation Non-treatment |
|
Delayed action |
Lack of results Re-investigation/ Consideration |
|
Lack of credibility |
Ignoring results Ignoring labo-ratory medicine |
Quality Assurance (QA) and Quality Control (QC) have always been important components of laboratory medicine. Quality assurance has been defined as "All those planned and systematic activities necessary to provide adequate confidence that a product or service will satisfy given requirements for quality". There are two types of quality asurance:
Internal: this is called QUALITY CONTROL. This means that each laboratory has a programme to check the quality of its own tests.
External: this is called QUALITY ASSESSMENT. This means that the laboratory performance is controlled by an external agency.
Therefore the need for laboratory accreditation on an international level has led to the development of External Quality Assessment Schemes(EQAS) as well as the set of guidelines - the ISO series- by the International Organisation for Standardisation (IOS). The latter establishes a framework for a quality-assurance system and unifies and standardises all QA activities. Also, EQAS provides assessment of: interlaboratory agreement, analytical procedures, individual laboratory performance and materials distributed.
In his lecture, Mr Buddoo, Director of MAURITAS, talked about the importance of accreditation and the possible benefits that can be derived thereby. He emphasised on the laboratory accreditation to ISO Guide 25 which by the end of this year will be known as ISO 17025. ISO Guide 25 is a recognition of laboratory competence whilst as compared to ISO 9000 which is simply a recognition of conformance to a quality system.
Dr Hemraj, Principal Biochemist, explained the concept of quality assurance as well as quality control. In order to contain the costs of quality standards, a proper quality management is needed and this is called Total Quality Management (TQM). He stressed that one of the critical points for good quality is the control of the pre-analytical step. In his exposé concerning quality control, he talked about the Westguard rules and the EQAS and said that quality assessment is broader than quality control and that quality improvement is the concept which reduces waste and increases quality.
The next lecturer, Mr Atchia from the Ministry of Environment explained how the environmental laboratories are targeting towards the ISO Guide 25 certification and how it can be achieved. Hence, an ISO 25 assessor is assigned to perform assessments only in their specific area of expertise.
A competent laboratory will have to go through the ISO 25 standard section by section, and wherever the standard states that an activity "shall" be performed, the laboratory makes sure to perform the procedure or activity as recommended. A typical assessment would consist of the assessor:
Mr Bundhoo, Senior Medical Laboratory Technician, talked about quality assurance at the Blood Tranfusion Service (BTS). He said that quality is important because the service must provide safest blood and blood products. Concerning the BTS, he explained what the goals of Quality assurance are as well as the quality control procedures.
At the question time, many issues were raised. The participants asked Mr Budoo about the possibility for accreditation of the lab for only one test. He replied positively and added that accreditation can be given to the different sections of one laboratory one by one. Then the logo of MAURITAS will only be used when eight tests out of ten are accreditated.
Another pertinent question was about patient preparation. Dr Hemraj replied that a user handbook should be made available to the respective department for specific tests such as Glucose Tolerance Test.. One participant from the private sector addressed a question to Dr Hemraj about the possibility of extending specimens for EQAS to the private laboratories. To him, Dr Hemraj replied that he had to contact the laboratories in UK for such a request and pointed out that the number of laboratories participating in such schemes must be statistically significant.
Then the principal biochemist said that the Central Health Laboratory could become a reference laboratory and that the right policy is needed. Responding to another question, Dr Hemraj said that in order to minimise the errors associated with pre-analytical variables, bar coding should be introduced.
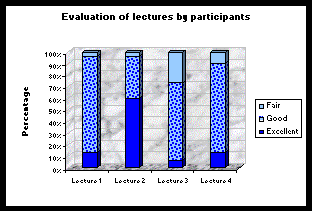
At the end of the four lectures, ABA carried out a survey among the participants. They evaluated the lecture of Dr Hemraj as excellent by 60% as compared to 13% for
Mr Budoo, 13% for Mr Bundhoo and 7% for Mr Atchia as shown in the graph below:

To meet the standards of excellence necessary for clinical laboratories, everyone in the organisation must accept responsibility for participating in providing the highest quality products and services. In the past, laboratory quality has been concentrated on the analytical process. But, now laboratory professionals accept that quality control of pre- and post analytical procedures reflect both a high level of service and exceptional laboratory quality. The latter will be expected by patients and doctors who depend on quality results in making life and death decisions.
Rehana Jauhangeer